WHY HYDROGEN IS A NEW DEMAND DRIVER FOR PLATINUM
The need to decarbonise is more acute than ever and platinum-based technologies have a significant role to play in the energy transition.
AUTOMOTIVE
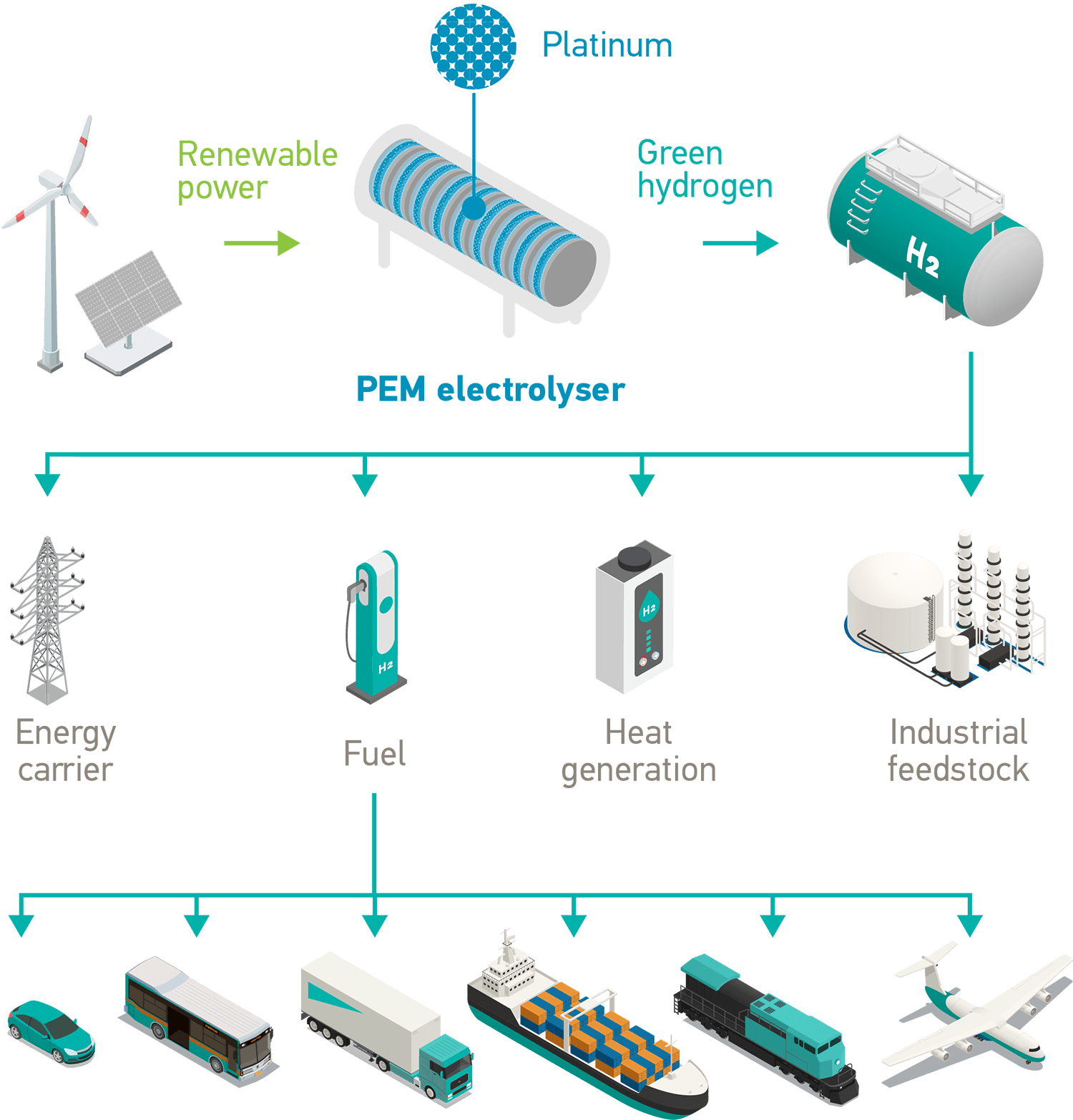
Platinum unlocks the hydrogen economy
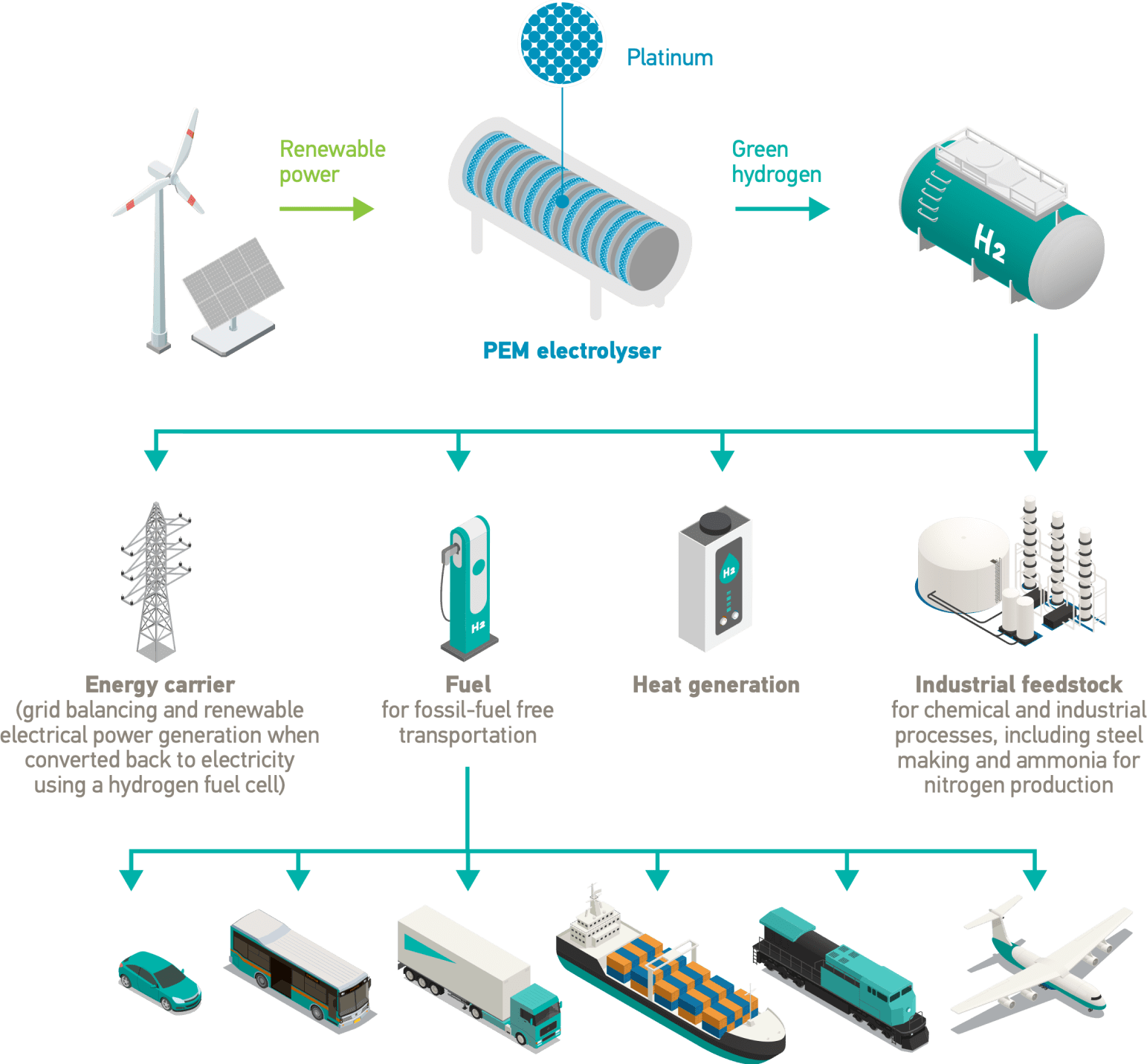
Proton exchange membrane (PEM) technology uses platinum catalysts in two key applications – electrolysers and hydrogen (H2) fuel cells to produce electricity. Fuel cell electric vehicles (FCEVs) are a major market for hydrogen fuel cells.
A PEM electrolyser produces carbon-free green hydrogen from renewable energy. If a FCEV is powered with green hydrogen it provides completely emissions-free transportation.
Platinum is a critical metal for the energy transition
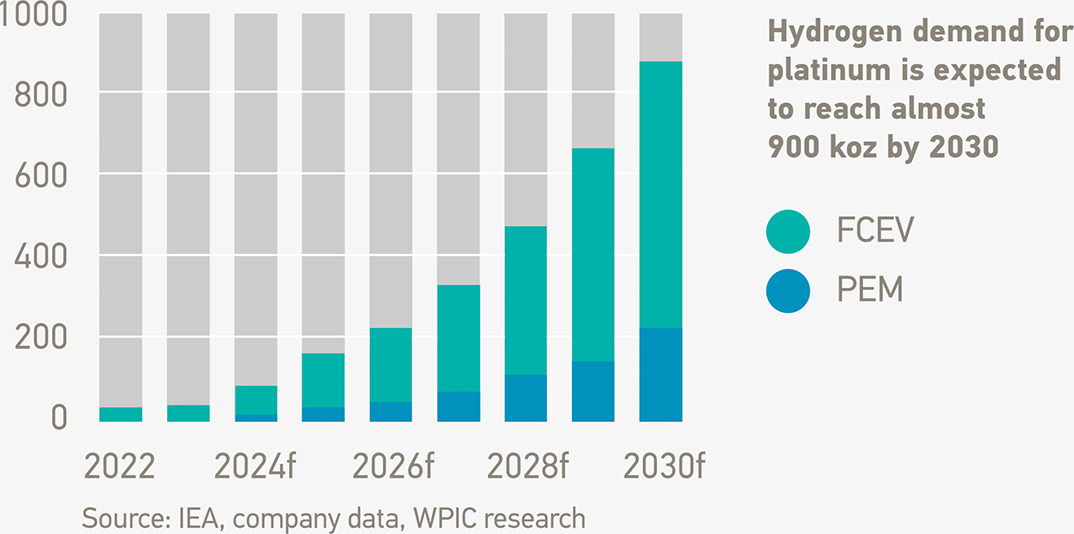
With hydrogen, platinum demand is benefiting from a major new emerging end-market
Platinum demand from PEM electrolysers and hydrogen fuel cells becomes a meaningful component of global platinum demand by 2030, reaching almost 900 koz.
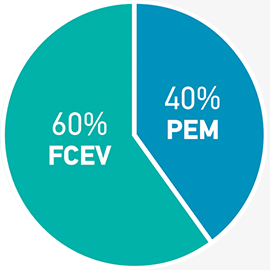
Fuel cells used in both mobility (land, sea and air transport) and stationary applications comprise the largest segment of projected hydrogen-related platinum demand, reaching over 600 koz by 2030.


PEM ELECTROLYSER
Platinum is crucial to PEM electrolysers
In an electrolyser electricity is used to break water into hydrogen and oxygen in a process called electrolysis. If the electricity comes from renewable sources the hydrogen produced is green hydrogen.
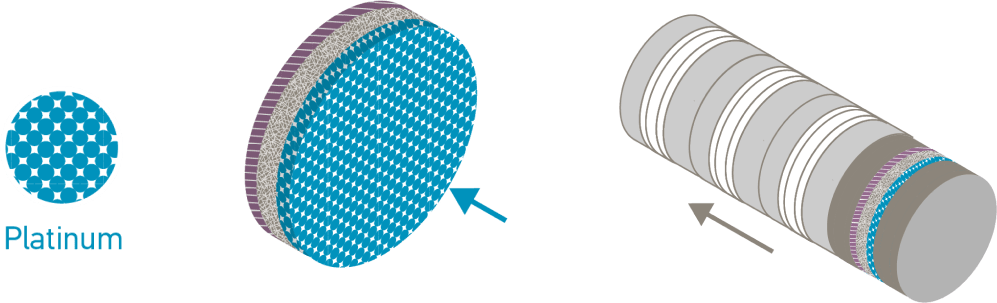
An electrolyser converts electrical energy into chemical energy, or electrons into molecules. PEM electrolysers harness the catalytic properties of platinum and its sister metal iridium. The platinum catalyst enables the splitting of the water into its constituent parts, providing a highly reactive surface area that can withstand corrosive conditions.
The PEM is coated with platinum at the cathode and iridium at the anode to make the catalyst coated membrane. Electrolysers can be scaled by combining individual cells to form an electrolyser stack, enabling multi-megawatt electrolyser installations.
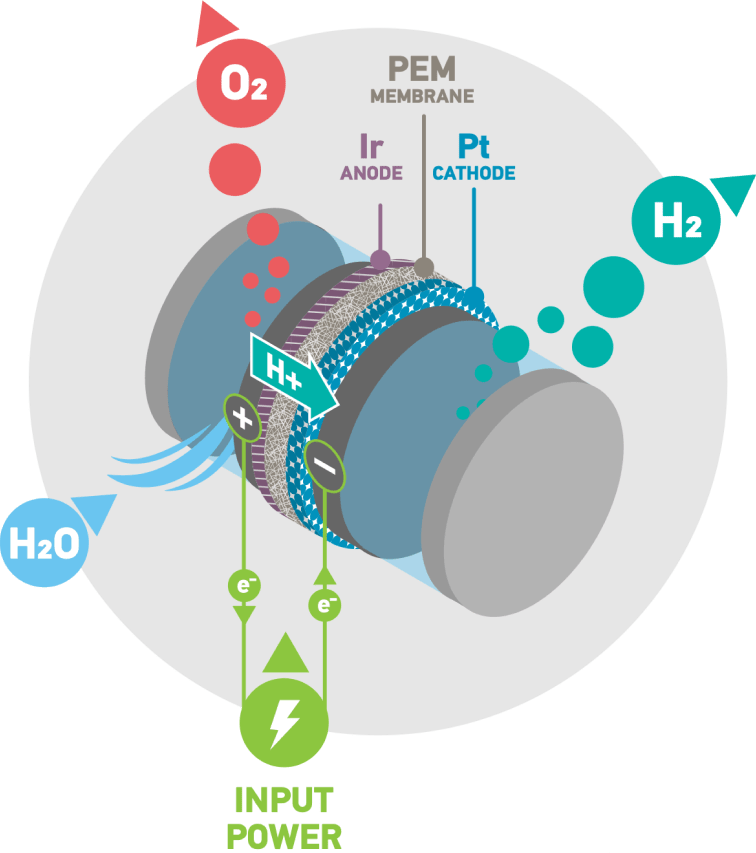
Inside a PEM electrolyser
PEM FUEL CELL
Over forty years of proven technology
Platinum is the catalyst that is used in PEM fuel cells as it provides the durability, stability and reactivity necessary to strip the hydrogen of electrons to produce electricity, leaving the hydrogen protons to pass through the PEM.
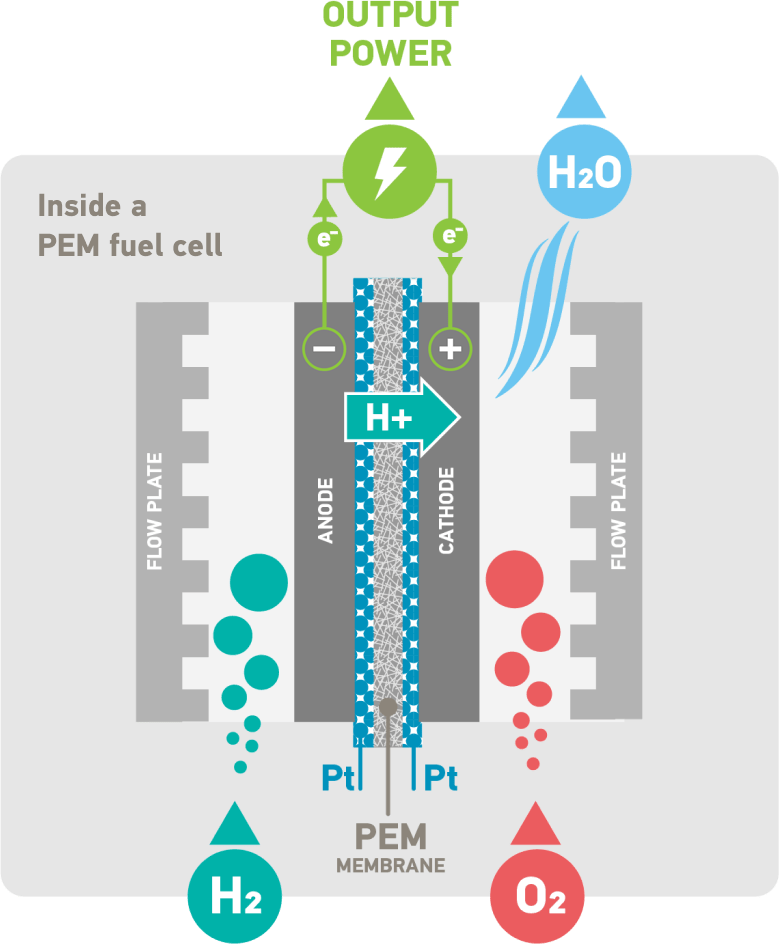
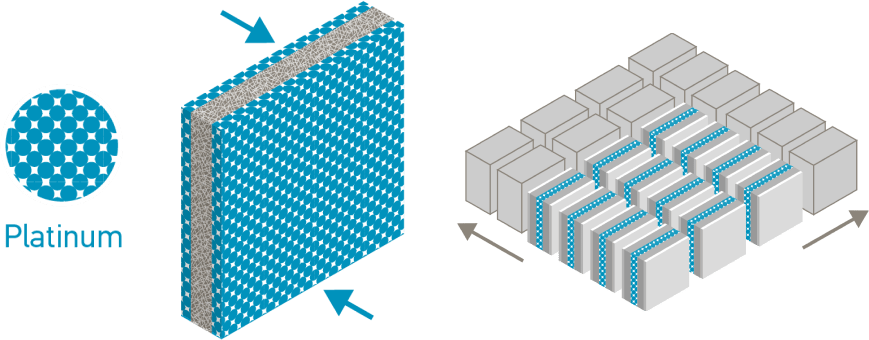
The PEM membrane is coated on both sides with a platinum catalyst. Platinum’s superior catalytic and conductive properties turn hydrogen and oxygen (from air) into electricity, with water and heat as the only by-products. A single fuel cell alone only produces a few watts of power, so multiple fuel cells are combined to create the right electric output, from a few kilowatts to multi-megawatt installations.
Hydrogen fuel cells provide emissions-free power – providing an alternative to battery electric solutions as a way of electrifying the global fleet of vehicles. Fuel cells in heavy-duty vehicles such as trucks and buses are currently leading the growing market for FCEVs.
PEM fuel cells can also be used to provide stationary or back-up power in, for example, data centres or for cell phone masts.
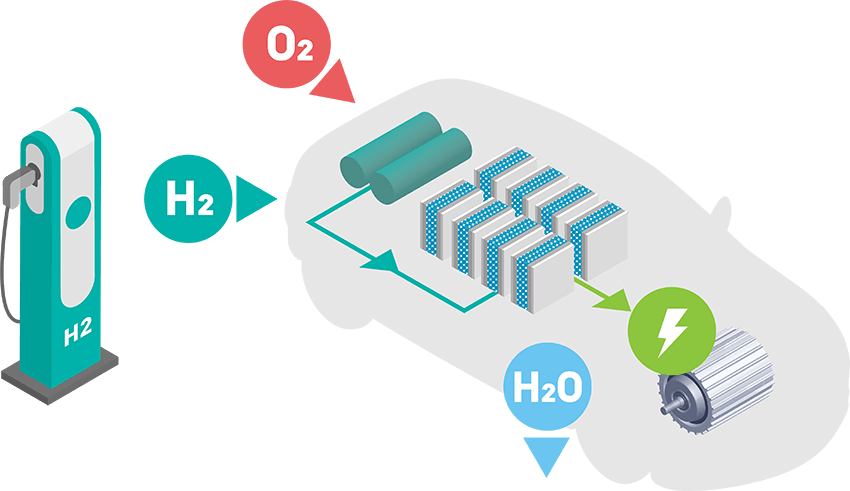
Fuel cell electric vehicle
MARKETS
Markets for platinum-based PEM technology are growing rapidly
Hydrogen will play a pivotal role in efforts to reach net zero, and investment, collaboration and the roll-out of supportive government policies are intensifying in order to achieve this, directly benefiting platinum demand.