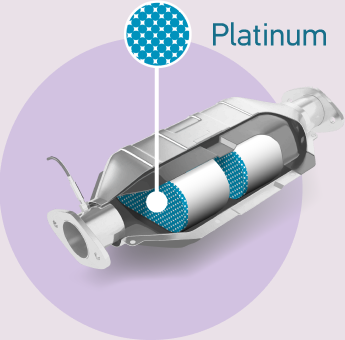
Platinum-based knitted gauzes are the most efficient catalysts for the production of nitric oxide as a precursor to the manufacture of nitric acid, the majority of which is used as a feedstock in the production of fertiliser.

How much do you know about platinum?
Rarer than gold, it is renowned as both a precious and industrial metal. What is more, over 80% of platinum's end-uses reduce energy requirements and/or harmful emissions.
Read our platinum facts to find out more.
Platinum-based knitted gauzes are the most efficient catalysts for the production of nitric oxide as a precursor to the manufacture of nitric acid, the majority of which is used as a feedstock in the production of fertiliser.

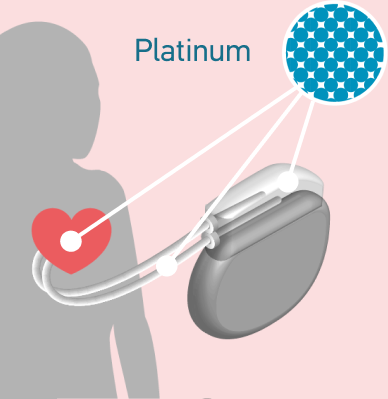
Platinum is the only material suitable for the electrodes required in the one million pacemakers implanted each year.
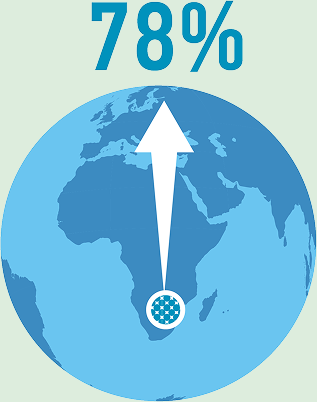
78% of platinum group metal reserves are concentrated in Southern Africa.
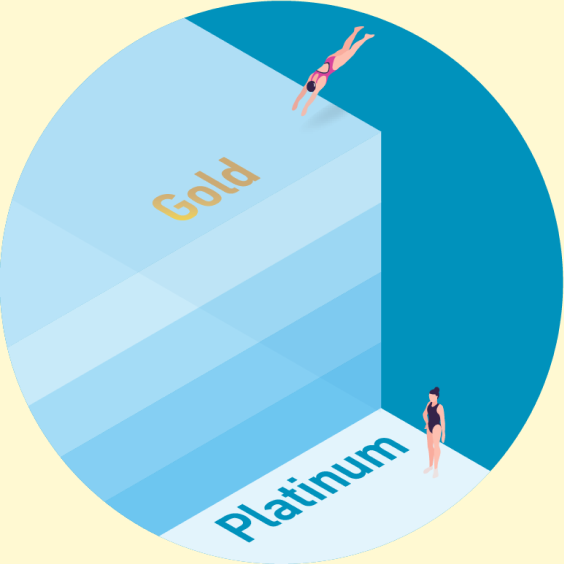
All the platinum ever produced would only cover your ankles in one Olympic-sized swimming pool. All the gold ever produced would fill four Olympic-sized swimming pools.
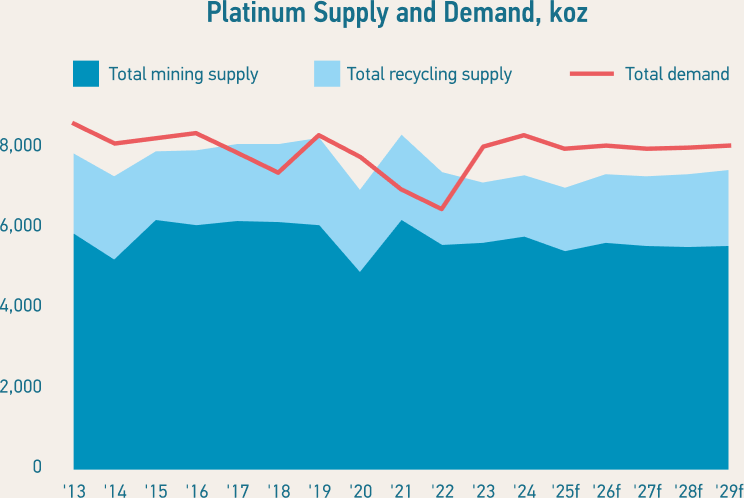
Global reserves of platinum are not at risk of running out this century, but the quantity of platinum mined each year cannot be easily or quickly increased to meet demand. Platinum can be recycled and this provides another supply source.
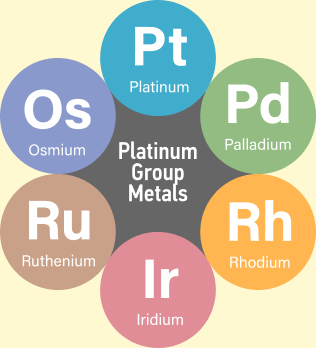
The six platinum group metals are platinum, palladium, rhodium, iridium, ruthenium and osmium.
Platinum is the rarest precious metal in the world with annual availability 20 times less than gold and 100 times less than silver.
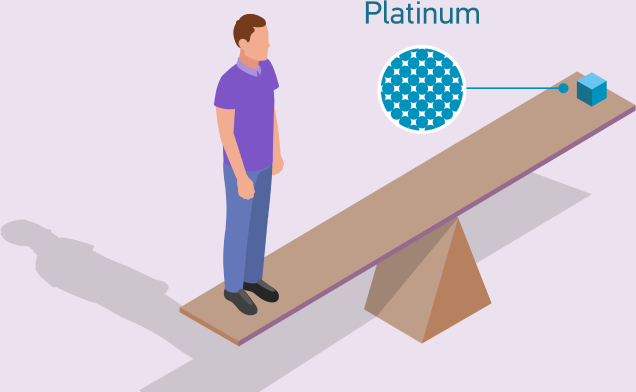
Platinum is an undervalued investment asset with strong demand and a shortage of supply.
A 15cm cube of platinum weighs as much as an average human being. Platinum is also one of the densest elements at 21.45g/cm3.
Platinum is highly malleable, meaning it can be pounded into a sheet which is as thin as 100 atoms.
Platinum continues to play a critical role in controlling harmful vehicle emissions.
The renowned Tiffany® Setting was developed in 1886. Its instantly recognisable six platinum prongs provide the security required for diamonds, while lifting them off the band into the light for maximum lustre. Today, versions of this setting are fabricated by jewellers worldwide.

Platinum was first used for coins in Russia in 1828.

At 1,768°C, platinum’s melting point is considerably higher than that of gold, which is 1,063°C.
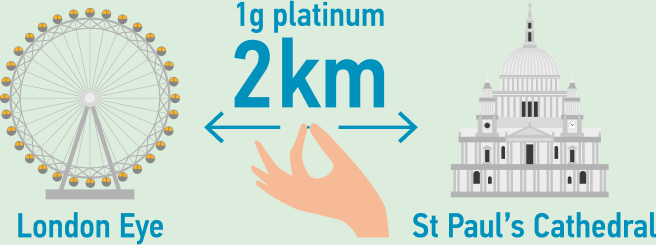
One gram of platinum can be stretched into a wire that is over 2 km long.
Typically 95% pure, platinum jewellery is one of the purest of all precious metals.

Platinum investment products are readily available. Investors can purchase physical platinum such as bars and coins as well as physically-backed investment products like vaulted platinum and Exchange Traded Funds (ETFs).

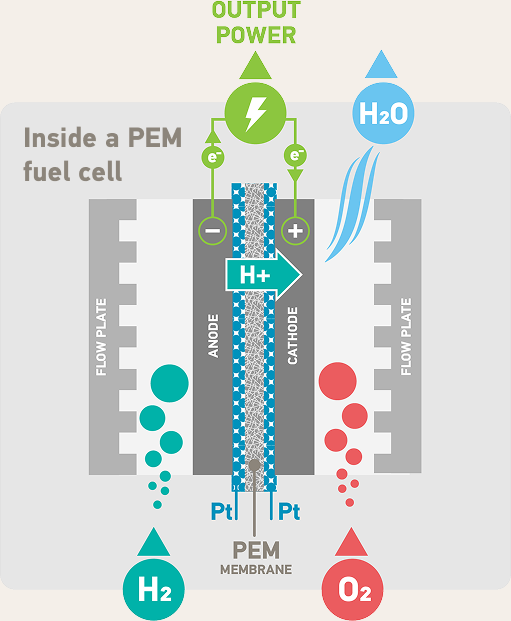
Platinum is responding to some of mankind’ s greatest challenges. Today, platinum catalysts are at the forefront of hydrogen-related technologies that are contributing to the energy transition.
Many countries, including the US, China and the UK , as well as the EU, include platinum on their critical minerals of strategic importance list.